Targeting sodium channel trafficking in cardiomyocytes to improve cardiac conduction
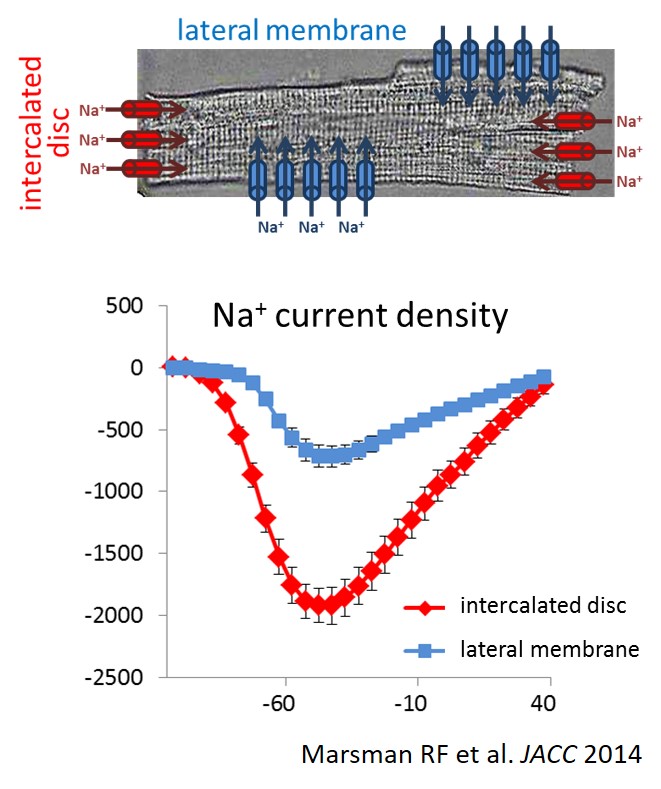
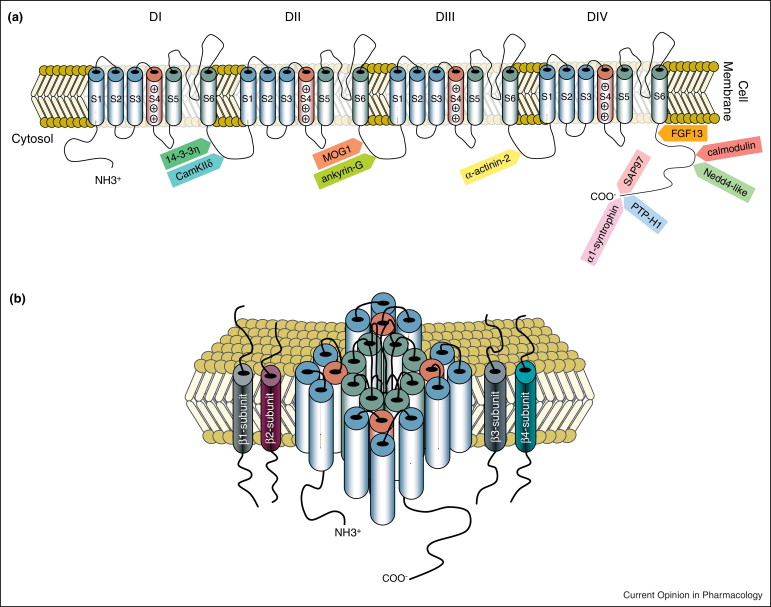
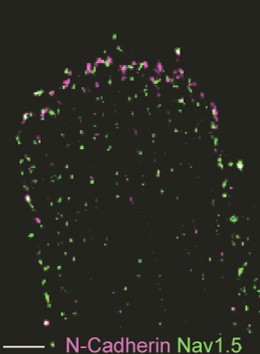
Distinct subcellular pools of Nav1.5-based sodium channels within the cardiomyocyte have been demonstrated, in particular at the intercalated disc and lateral membrane regions. Within these domains, Nav1.5 channels are not homogeneously distributed but rather grouped in clusters of various sizes and densities. Furthermore, different interacting proteins associate with Nav1.5 at these distinct subcellular domains, and are thought to contribute to the differences in channel density and/or kinetics observed between these areas. Sodium channels located at the lateral membrane are associated with the syntrophin-dystrophin complex. We have previously shown that mice lacking the last three amino acids of Nav1.5 essential for the interaction with the syntrophin-dystrophin complex show sodium current reduction exclusively at the lateral membrane (Shy et al. Circulation 2014). Nav1.5 channels in the intercalated disc region (which is devoid of syntrophin) interact with SAP97, plakophilin-2, desmoglein-2 and coxsackie and adenovirus receptor (CAR).
We have demonstrated that in mice with reduced expression of CAR (a cell adhesion protein enriched at the intercalated disc), sodium current was preferentially reduced at the intercalated disc region associated with increased arrhythmia inducibility during myocardial ischemia (Marsman et al. JACC 2014). Overall, little is still known about the specific roles for these separate pools of channels or their functional relevance during (patho)physiological conditions. Moreover, the consequences of subcellular diversity in sodium channel composition and function for disease severity and expressivity of SCN5A mutations form a challenging topic for future investigations.
Our current work is furthermore aimed at unraveling the trafficking pathways through which sodium channels reach their various subcellular destinations in the cardiomyocyte, in order to identify novel therapeutic strategies to increase sodium current and rescue cardiac conduction.
Group members
Carol Ann Remme
Group Leader, PI
Vincent Portero
Post Doc
Simona Casini
Post Doc
Giovanna Nasilli
PhD Student
Gerard Marchal
PhD Student